The unique::Eng:: Ahmed Gamal Ahmed ::::culture and news with business in gold , oil SPORT , petroleum , hosting , computer , free games , technology , engineering , mechanical engineering , education , software , hardware , marketing , dollar and gold.

Custom Search
Dec 31, 2010
Electronics
The concentration of free electrons in gold metal is 5.90×1022 cm−3. Gold is highly conductive to electricity, and has been used for electrical wiring in some high-energy applications (only silver and copper are more conductive per volume, but gold has the advantage of corrosion resistance). For example, gold electrical wires were used during some of the Manhattan Project's atomic experiments, but large high current silver wires were used in the calutron isotope separator magnets in the project.Though gold is attacked by free chlorine, its good conductivity and general resistance to oxidation and corrosion in other environments (including resistance to non-chlorinated acids) has led to its widespread industrial use in the electronic era as a thin layer coating electrical connectors of all kinds, thereby ensuring good connection. For example, gold is used in the connectors of the more expensive electronics cables, such as audio, video and USB cables. The benefit of using gold over other connector metals such as tin in these applications is highly debated. Gold connectors are often criticized by audio-visual experts as unnecessary for most consumers and seen as simply a marketing ploy. However, the use of gold in other applications in electronic sliding contacts in highly humid or corrosive atmospheres, and in use for contacts with a very high failure cost (certain computers, communications equipment, spacecraft, jet aircraft engines) remains very common.[30]
Besides sliding electrical contacts, gold is also used in electrical contacts because of its resistance to corrosion, electrical conductivity, ductility and lack of toxicity.[31] Switch contacts are generally subjected to more intense corrosion stress than are sliding contacts. Fine gold wires are used to connect semiconductor devices to their packages through a process known as wire bonding.
Commercial chemistry
Gold is attacked by and dissolves in alkaline solutions of potassium or sodium cyanide, to form the salt gold cyanide—a technique that has been used in extracting metallic gold from ores in the cyanide process. Gold cyanide is the electrolyte used in commercial electroplating of gold onto base metals and electroforming.Gold chloride (chloroauric acid) solutions are used to make colloidal gold by reduction with citrate or ascorbate ions. Gold chloride and gold oxide are used to make highly valued cranberry or red-colored glass, which, like colloidal gold suspensions, contains evenly sized spherical gold nanoparticles.[32]
History
Gold has been known and used by artisans since the Chalcolithic. Gold artifacts in the Balkans appear from the 4th millennium BC, such as that found in the Varna Necropolis. Gold artifacts such as the golden hats and the Nebra disk appeared in Central Europe from the 2nd millennium BC Bronze Age.Egyptian hieroglyphs from as early as 2600 BC describe gold, which king Tushratta of the Mitanni claimed was "more plentiful than dirt" in Egypt.[33] Egypt and especially Nubia had the resources to make them major gold-producing areas for much of history. The earliest known map is known as the Turin Papyrus Map and shows the plan of a gold mine in Nubia together with indications of the local geology. The primitive working methods are described by both Strabo and Diodorus Siculus, and included fire-setting. Large mines were also present across the Red Sea in what is now Saudi Arabia.
The legend of the golden fleece may refer to the use of fleeces to trap gold dust from placer deposits in the ancient world. Gold is mentioned frequently in the Old Testament, starting with Genesis 2:11 (at Havilah) and is included with the gifts of the magi in the first chapters of Matthew New Testament. The Book of Revelation 21:21 describes the city of New Jerusalem as having streets "made of pure gold, clear as crystal". The south-east corner of the Black Sea was famed for its gold. Exploitation is said to date from the time of Midas, and this gold was important in the establishment of what is probably the world's earliest coinage in Lydia around 610 BC.[34] From the 6th or 5th century BC, the Chu (state) circulated the Ying Yuan, one kind of square gold coin.
In Roman metallurgy, new methods for extracting gold on a large scale were developed by introducing hydraulic mining methods, especially in Hispania from 25 BC onwards and in Dacia from 106 AD onwards. One of their largest mines was at Las Medulas in León (Spain), where seven long aqueducts enabled them to sluice most of a large alluvial deposit. The mines at Roşia Montană in Transylvania were also very large, and until very recently, still mined by opencast methods. They also exploited smaller deposits in Britain, such as placer and hard-rock deposits at Dolaucothi. The various methods they used are well described by Pliny the Elder in his encyclopedia Naturalis Historia written towards the end of the first century AD.
The Mali Empire in Africa was famed throughout the old world for its large amounts of gold. Mansa Musa, ruler of the empire (1312–1337) became famous throughout the old world for his great hajj to Mecca in 1324. When he passed through Cairo in July 1324, he was reportedly accompanied by a camel train that included thousands of people and nearly a hundred camels. He gave away so much gold that it depressed the price in Egypt for over a decade.[35] A contemporary Arab historian remarked:
| “ | Gold was at a high price in Egypt until they came in that year. The mithqal did not go below 25 dirhams and was generally above, but from that time its value fell and it cheapened in price and has remained cheap till now. The mithqal does not exceed 22 dirhams or less. This has been the state of affairs for about twelve years until this day by reason of the large amount of gold which they brought into Egypt and spent there [...] | ” |
Although the price of some platinum group metals can be much higher, gold has long been considered the most desirable of precious metals, and its value has been used as the standard for many currencies (known as the gold standard) in history. Gold has been used as a symbol for purity, value, royalty, and particularly roles that combine these properties. Gold as a sign of wealth and prestige was ridiculed by Thomas More in his treatise Utopia. On that imaginary island, gold is so abundant that it is used to make chains for slaves, tableware and lavatory-seats. When ambassadors from other countries arrive, dressed in ostentatious gold jewels and badges, the Utopians mistake them for menial servants, paying homage instead to the most modestly dressed of their party.
There is an age-old tradition of biting gold to test its authenticity. Although this is certainly not a professional way of examining gold, the bite test should score the gold because gold is a soft metal, as indicated by its score on the Mohs' scale of mineral hardness. The purer the gold the easier it should be to mark it. Painted lead can cheat this test because lead is softer than gold (and may invite a small risk of lead poisoning if sufficient lead is absorbed by the biting).
Gold in antiquity was relatively easy to obtain geologically; however, 75% of all gold ever produced has been extracted since 1910.[38] It has been estimated that all gold ever refined would form a single cube 20 m (66 ft) on a side (equivalent to 8000 m3).[38]
One main goal of the alchemists was to produce gold from other substances, such as lead — presumably by the interaction with a mythical substance called the philosopher's stone. Although they never succeeded in this attempt, the alchemists promoted an interest in what can be done with substances, and this laid a foundation for today's chemistry. Their symbol for gold was the circle with a point at its center (☉), which was also the astrological symbol and the ancient Chinese character for the Sun. For modern creation of artificial gold by neutron capture, see gold synthesis.
During the 19th century, gold rushes occurred whenever large gold deposits were discovered. The first documented discovery of gold in the United States was at the Reed Gold Mine near Georgeville, North Carolina in 1803.[39] The first major gold strike in the United States occurred in a small north Georgia town called Dahlonega.[40] Further gold rushes occurred in California, Colorado, the Black Hills, Otago in New Zealand, Australia, Witwatersrand in South Africa, and the Klondike in Canada.
Because of its historically high value, much of the gold mined throughout history is still in circulation in one form or another.
Occurrence

This 156-ounce (4.85 kg) nugget was found by an individual prospector in the Southern California Desert using a metal detector.
On Earth, whenever elemental gold occurs, it appears most often as a metal solid solution of gold with silver, i.e. a gold silver alloy. Such alloys usually have a silver content of 8–10%. Electrum is elemental gold with more than 20% silver. Electrum's color runs from golden-silvery to silvery, dependent upon the silver content. The more silver, the lower the specific gravity.

Relative sizes of an 860 kg block of gold ore, and the 30 g of gold that can be extracted from it. Toi gold mine, Japan.
Gold sometimes occurs combined with tellurium as the minerals calaverite, krennerite, nagyagite, petzite and sylvanite, and as the rare bismuthide maldonite (Au2Bi) and antimonide aurostibite (AuSb2). Gold also occurs in rare alloys with copper, lead, and mercury: the minerals auricupride (Cu3Au), novodneprite (AuPb3) and weishanite ((Au, Ag)3Hg2).
Recent research suggests that microbes can sometimes play an important role in forming gold deposits, transporting and precipitating gold to form grains and nuggets that collect in alluvial deposits.[43]
The world's oceans contain gold. Measured concentrations of gold in the Atlantic and Northeast Pacific are 50–150 fmol/L or 10-30 parts per quadrillion. In general, Au concentrations for Atlantic and Pacific samples are the same (~50 fmol/L) but less certain. Mediterranean deep waters contain higher concentrations of Au (100–150 fmol/L) attributed to wind-blown dust and/or rivers. At 10 parts per quadrillion the Earth's oceans would hold 15,000 tons of gold.[44] These figures are three orders of magnitude less than reported in the literature prior to 1988, indicating contamination problems with the earlier data.
A number of people have claimed to be able to economically recover gold from sea water, but so far they have all been either mistaken or crooks. A so-called reverend, Prescott Jernegan ran a gold-from-seawater swindle in the United States in the 1890s. A British fraudster ran the same scam in England in the early 1900s.[45] Fritz Haber (the German inventor of the Haber process) did research on the extraction of gold from sea water in an effort to help pay Germany's reparations following World War I.[46] Based on the published values of 2 to 64 ppb of gold in seawater a commercially successful extraction seemed possible. After analysis of 4000 water samples yielding an average of 0.004 ppb it became clear that the extraction would not be possible and he stopped the project.[47] No commercially viable mechanism for performing gold extraction from sea water has yet been identified. Gold synthesis is not economically viable and is unlikely to become so in the foreseeable future
Gallery of specimens of crystalline native gold
| | Crystalline gold from Mina Zapata, Santa Elena de Uairen, Venezuela. Size: 3.7×1.1×0.4 cm. | Gold leaf from Harvard Mine, Jamestown, California, USA. Size 9.3×3.2× >0.1 cm. |
Production

Pure gold precipitate produced by the aqua regia refining process
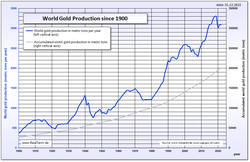
Since the 1880s, South Africa has been the source for a large proportion of the world's gold supply, with about 50% of all gold ever produced having come from South Africa. Production in 1970 accounted for 79% of the world supply, producing about 1,480 tonnes. 2008 production was 2,260 tonnes. In 2007 China (with 276 tonnes) overtook South Africa as the world's largest gold producer, the first time since 1905 that South Africa has not been the largest.[48]
The city of Johannesburg located in South Africa was founded as a result of the Witwatersrand Gold Rush which resulted in the discovery of some of the largest gold deposits the world has ever seen. Gold fields located within the basin in the Free State and Gauteng provinces are extensive in strike and dip requiring some of the world's deepest mines, with the Savuka and TauTona mines being currently the world's deepest gold mine at 3,777 m. The Second Boer War of 1899–1901 between the British Empire and the Afrikaner Boers was at least partly over the rights of miners and possession of the gold wealth in South Africa.
Other major producers are the United States, Australia, Russia and Peru. Mines in South Dakota and Nevada supply two-thirds of gold used in the United States. In South America, the controversial project Pascua Lama aims at exploitation of rich fields in the high mountains of Atacama Desert, at the border between Chile and Argentina. Today about one-quarter of the world gold output is estimated to originate from artisanal or small scale mining.[49]
After initial production, gold is often subsequently refined industrially by the Wohlwill process which is based on electrolysis or by the Miller process, that is chlorination in the melt. The Wohlwill process results in higher purity, but is more complex and is only applied in small-scale installations.[50][51] Other methods of assaying and purifying smaller amounts of gold include parting and inquartation as well as cupellation, or refining methods based on the dissolution of gold in aqua regia.[52]
At the end of 2009, it was estimated that all the gold ever mined totaled 165,000 tonnes[1] This can be represented by a cube with an edge length of about 20.28 meters. The value of this is very limited; at $1200 per ounce, 165,000 tons of gold would have a value of only 6.6 trillion dollars.
The average gold mining and extraction costs were about US$317/oz in 2007, but these can vary widely depending on mining type and ore quality; global mine production amounted to 2,471.1 tonnes.[53]
Gold is so stable and so valuable that most of the gold used in manufactured goods, jewelry, and works of art is eventually recovered and recycled. Some gold used in spacecraft and electronic equipment cannot be profitably recovered, but it is generally used in these applications in the form of extremely thin layers or extremely fine wires so that the total quantity used (and lost) is small compared to the total amount of gold produced and stockpiled. Thus there is little true consumption of new gold in the economic sense; the stock of gold remains essentially constant (at least in the modern world) while ownership shifts from one party to another.[54] One estimate is that 85% of all the gold ever mined is still available in the world's easily recoverable stocks, with 15% having been lost, or used in non-recyclable industrial uses.[55]
Consumption
The consumption of gold produced in the world is about 50% in jewelry, 40% in investments, and 10% in industry.India is the world's largest single consumer of gold, as Indians buy about 25% of the world's gold,[56] purchasing approximately 800 tonnes of gold every year, mostly for jewelry. India is also the largest importer gold; in 2008, India imported around 400 tonnes of gold.[57]
Chemistry
Although gold is a noble metal, it forms many and diverse compounds. The oxidation state of gold in its compounds ranges from −1 to +5, but Au(I) and Au(III) dominate its chemistry. Au(I), referred to as the aurous ion, is the most common oxidation state with soft ligands such as thioethers, thiolates, and tertiary phosphines. Au(I) compounds are typically linear. A good example is Au(CN)2−, which is the soluble form of gold encountered in mining. Curiously, aurous complexes of water are rare. The binary gold halides, such as AuCl, form zigzag polymeric chains, again featuring linear coordination at Au. Most drugs based on gold are Au(I) derivatives.[58]Au(III) (auric) is a common oxidation state, and is illustrated by gold(III) chloride, Au2Cl6. The gold atom centers in Au(III) complexes, like other d8 compounds, are typically square planar, with chemical bonds that have both covalent and ionic character.
Aqua regia, a 1:3 mixture of nitric acid and hydrochloric acid, dissolves gold. Nitric acid oxidizes the metal to +3 ions, but only in minute amounts, typically undetectable in the pure acid because of the chemical equilibrium of the reaction. However, the ions are removed from the equilibrium by hydrochloric acid, forming AuCl4− ions, or chloroauric acid, thereby enabling further oxidation.
Some free halogens react with gold.[59] Gold also reacts in alkaline solutions of potassium cyanide. With mercury, it forms an amalgam.
Less common oxidation states
Less common oxidation states of gold include −1, +2, and +5.The −1 oxidation state occurs in compounds containing the Au− anion, called aurides. Caesium auride (CsAu), for example, crystallizes in the caesium chloride motif.[60] Other aurides include those of Rb+, K+, and tetramethylammonium (CH3)4N+.[61]
Gold(II) compounds are usually diamagnetic with Au–Au bonds such as [Au(CH2)2P(C6H5)2]2Cl2. The evaporation of a solution of Au(OH)3 in concentrated H2SO4 produces red crystals of gold(II) sulfate, AuSO4. Originally thought to be a mixed-valence compound, it has been shown to contain Au4+
2 cations.[62][63] A noteworthy, legitimate gold(II) complex is the tetraxenonogold(II) cation, which contains xenon as a ligand, found in [AuXe4](Sb2F11)2.[64]
Gold pentafluoride and its derivative anion, AuF−
6, is the sole example of gold(V), the highest verified oxidation state.[65]
Some gold compounds exhibit aurophilic bonding, which describes the tendency of gold ions to interact at distances that are too long to be a conventional Au–Au bond but shorter that van der Waals bonding. The interaction is estimated to be comparable in strength to that of a hydrogen bond.
Mixed valence compounds
Well-defined cluster compounds are numerous.[61] In such cases, gold has a fractional oxidation state. A representative example is the octahedral species {Au(P(C6H5)3)}62+. Gold chalcogenides, such as gold sulfide, feature equal amounts of Au(I) and Au(III).Toxicity
Pure metallic (elemental) gold is non-toxic and non-irritating when ingested[66] and is sometimes used as a food decoration in the form of gold leaf. Metallic gold is also a component of the alcoholic drinks Goldschläger, Gold Strike, and Goldwasser. Metallic gold is approved as a food additive in the EU (E175 in the Codex Alimentarius). Although gold ion is toxic, the acceptance of metallic gold as a food additive is due to its relative chemical inertness, and resistance to being corroded or transformed into soluble salts (gold compounds) by any known chemical process which http://www.blogger.com/post-create.g?blogID=2546579627818874895would be encountered in the human body.Soluble compounds (gold salts) such as gold chloride are toxic to the liver and kidneys. Common cyanide salts of gold such as potassium gold cyanide, used in gold electroplating, are toxic both by virtue of their cyanide and gold content. There are rare cases of lethal gold poisoning from potassium gold cyanide.[67][68] Gold toxicity can be ameliorated with chelation therapy with an agent such as Dimercaprol.
Gold metal was voted Allergen of the Year in 2001 by the American Contact Dermatitis Society. Gold contact allergies affect mostly women.[69] Despite this, gold is a relatively non-potent contact allergen, in comparison with metals like nickel.[70]
Labels:
Mechanical engineering
Price

Gold price per troy ounce in USD since 1960, in nominal US$ (black) and in 2009 US$ (red) after inflation adjustment using the CPI-U price index.
The price of gold is determined through trading in the gold and derivatives markets, but a procedure known as the Gold Fixing in London, originating in September 1919, provides a daily benchmark price to the industry. The afternoon fixing was introduced in 1968 to provide a price when US markets are open.
Historically gold coinage was widely used as currency; when paper money was introduced, it typically was a receipt redeemable for gold coin or bullion. In a monetary system known as the gold standard, a certain weight of gold was given the name of a unit of currency. For a long period, the United States government set the value of the US dollar so that one troy ounce was equal to $20.67 ($664.56/kg), but in 1934 the dollar was devalued to $35.00 per troy ounce ($1125.27/kg). By 1961, it was becoming hard to maintain this price, and a pool of US and European banks agreed to manipulate the market to prevent further currency devaluation against increased gold demand.

Swiss-cast 1 kg gold bar
In 2005 the World Gold Council estimated total global gold supply to be 3,859 tonnes and demand to be 3,754 tonnes, giving a surplus of 105 tonnes.[71]
Since 1968 the price of gold has ranged widely, from a high of $850/oz ($27,300/kg) on January 21, 1980, to a low of $252.90/oz ($8,131/kg) on June 21, 1999 (London Gold Fixing).[72] The period from 1999 to 2001 marked the "Brown Bottom" after a 20-year bear market.[73] Prices increased rapidly from 1991, but the 1980 high was not exceeded until January 3, 2008 when a new maximum of $865.35 per troy ounce was set (a.m. London Gold Fixing).[74] Another record price was set on March 17, 2008 at $1023.50/oz ($32,900/kg)(am. London Gold Fixing).[74] In the fall of 2009, gold markets experienced renewed momentum upwards due to increased demand and a weakening US dollar. On December 2, 2009, Gold passed the important barrier of US$1200 per ounce to close at $1215.[75] Gold further rallied hitting new highs in May 2010 after the European Union debt crisis prompted further purchase of gold as a safe asset.[76][77]
Since April 2001 the gold price has more than tripled in value against the US dollar,[78] prompting speculation that this long secular bear market has ended and a bull market has returned.[79]
Symbolism

Gold bars at the Emperor Casino in Macau
Great human achievements are frequently rewarded with gold, in the form of gold medals, golden trophies and other decorations. Winners of athletic events and other graded competitions are usually awarded a gold medal (e.g., the Olympic Games). Many awards such as the Nobel Prize are made from gold as well. Other award statues and prizes are depicted in gold or are gold plated (such as the Academy Awards, the Golden Globe Awards, the Emmy Awards, the Palme d'Or, and the British Academy Film Awards).
Aristotle in his ethics used gold symbolism when referring to what is now commonly known as the "golden mean". Similarly, gold is associated with perfect or divine principles, such as in the case of Phi, which is sometimes called the "golden ratio".
Gold represents great value. Respected people are treated with the most valued rule, the "golden rule". A company may give its most valued customers "gold cards" or make them "gold members". We value moments of peace and therefore we say: "silence is golden". In Greek mythology there was the "golden fleece".
Gold is further associated with the wisdom of aging and fruition. The fiftieth wedding anniversary is golden. Our precious latter years are sometimes considered "golden years". The height of a civilization is referred to as a "golden age".
In Christianity gold has sometimes been associated with the extremities of utmost evil and the greatest sanctity. In the Book of Exodus, the Golden Calf is a symbol of idolatry. In the Book of Genesis, Abraham was said to be rich in gold and silver, and Moses was instructed to cover the Mercy Seat of the Ark of the Covenant with pure gold. In Christian art the halos of Christ, Mary and the Christian saints are golden.
Medieval kings were inaugurated under the signs of sacred oil and a golden crown, the latter symbolizing the eternal shining light of heaven and thus a Christian king's divinely inspired authority. Wedding rings have long been made of gold. It is long lasting and unaffected by the passage of time and may aid in the ring symbolism of eternal vows before God and/or the sun and moon and the perfection the marriage signifies. In Orthodox Christianity, the wedded couple is adorned with a golden crown during the ceremony, an amalgamation of symbolic rites.
In popular culture gold holds many connotations but is most generally connected to terms such as good or great, such as in the phrases: "has a heart of gold", "that's golden!", "golden moment", "then you're golden!" and "golden boy". Gold also still holds its place as a symbol of wealth and through that, in many societies, success.
State emblem
In 1965, the California Legislature designated gold "the State Mineral and mineralogical emblem."[80]In 1968, the Alaska Legislature named gold "the official state mineral."[81]
See also
References
- ^ a b World Gold Council FAQ
- ^ "Gold: causes of color". http://www.webexhibits.org/causesofcolor/9.html. Retrieved 2009-06-06.
- ^ Mallan, Lloyd (1971). Suiting up for space: the evolution of the space suit. John Day Co. p. 216. ISBN 978-0381981501.
- ^ a b "Gold Jewellery Alloys > Utilise Gold. Scientific, industrial and medical applications, products ,suppliers from the World Gold Council". Utilisegold.com. 2000-01-20. http://www.utilisegold.com/jewellery_technology/colours/colour_alloys/. Retrieved 2009-04-05.
- ^ Pelouze, Jules and Fremy, Edmond (1854). General notions of chemistry. Lippincott, Grambo & Co.. p. 280. http://books.google.com/?id=C8UHAAAAIAAJ&pg=PA280.
- ^ "Relativity in Chemistry". Math.ucr.edu. http://math.ucr.edu/home/baez/physics/Relativity/SR/gold_color.html. Retrieved 2009-04-05.
- ^ Schmidbaur, Hubert; Cronje, Stephanie; Djordjevic, Bratislav; Schuster, Oliver (2005). "Understanding gold chemistry through relativity". Chemical Physics 311 (1–2): 151–161. doi:10.1016/j.chemphys.2004.09.023.
- ^ National Nuclear Data Center Nudat 2
- ^ a b Audi, G. (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A (Atomic Mass Data Center) 729: 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ King, Byron (2009-07-20). "Gold mining decline". BullionVault.com. http://goldnews.bullionvault.com/gold_mine_production_072020092. Retrieved 2009-11-23.
- ^ "Gold Backed Currency - MoneyTec.com Traders Community Forum". Moneytec.com. http://www.moneytec.com/forums/f33/gold-backed-currency-14196/. Retrieved 2009-04-05.
- ^ Martin Feldstein (2009-12-26). "Is Gold a Good Hedge?". Project Syndicate. http://host.madison.com/ct/news/opinion/column/article_68f99b80-4258-5f44-a817-5cc64c6e1884.html. Retrieved 2009-12-29.
- ^ World Gold Council, Jewellery Technology, Jewellery Alloys
- ^ "The healing power of precious metals". http://health.ninemsn.com.au/article.aspx?id=694367. Retrieved 2009-06-06.
- ^ a b Messori, L.; Marcon, G. (2004). "Gold Complexes in the treatment of Rheumatoid Arthritis". In Sigel, Astrid. Metal ions and their complexes in medication. CRC Press. pp. 280–301. ISBN 9780824753511. http://books.google.com/?id=wgifUs8dFbgC&pg=PA279.
- ^ "BMJ: ''login required''". Besthealth.bmj.com. http://besthealth.bmj.com/btuk/conditions/14212.html. Retrieved 2009-04-05. [dead link]
- ^ Faulk, WP; Taylor, GM (1971). "An immunocolloid method for the electron microscope.". Immunochemistry 8 (11): 1081–3. doi:10.1016/0019-2791(71)90496-4. PMID 4110101.
- ^ Roth, J; Bendayan, M; Orci, L (1980). "FITC-protein A-gold complex for light and electron microscopic immunocytochemistry.". The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 28 (1): 55–7. PMID 6153194. http://www.jhc.org/cgi/reprint/28/1/55.pdf.
- ^ Bozzola, John J. and Russell, Lonnie Dee (1999). Electron microscopy: principles and techniques for biologists. Jones & Bartlett Learning. p. 65. ISBN 0763701920. http://books.google.com/?id=RqSMzR-IXk0C&pg=PA65.
- ^ Nanoscience and Nanotechnology in Nanomedicine: Hybrid Nanoparticles In Imaging and Therapy of Prostate Cancer - Radiopharmaceutical Sciences Institute, University of Missouri-Columbia
- ^ "Current EU approved additives and their E Numbers". Food Standards Agency, UK. 27 July 2007. http://www.food.gov.uk/safereating/chemsafe/additivesbranch/enumberlist.
- ^ "The Food Dictionary: Varak". Barron's Educational Services, Inc. 1995. http://www.epicurious.com/cooking/how_to/food_dictionary/entry?id=5061. Retrieved 2007-05-27.
- ^ Indian Recipes
- ^ Baedeker, Karl (1865). "Danzig" (in German). Deutschland nebst Theilen der angrenzenden Länder. Karl Baedeker. http://books.google.com/?id=tsUNAAAAYAAJ&pg=PA101.
- ^ Guiness Book of World Records 2008
- ^ "The Many Uses of Gold". http://geology.com/minerals/gold/uses-of-gold.shtml. Retrieved 2009-06-06.
- ^ Kodak (2006) Toning black-and-white materials. Technical Data/Reference sheet G-23, May 2006.
- ^ Super cars.net. 1994 McLaren F1
- ^ "The Demand for Gold by Industry". Gold bulletin. http://www.goldbulletin.org/assets/file/goldbulletin/downloads/Cooke_2_15.pdf. Retrieved 2009-06-06.
- ^ Krech, Shepard; McNeill, John Robert and Merchant, Carolyn (2004). Encyclopedia of world environmental history, Volume 3. Routledge. p. 597. ISBN 0415937345. http://books.google.com/?id=G7JrhAy5phoC&pg=PA597.
- ^ "General Electric Contact Materials". Electrical Contact Catalog (Material Catalog). Tanaka Precious Metals. 2005. http://www.tanaka-precious.com/catalog/material.html. Retrieved 2007-02-21.
- ^ "Colored glass chemistry". http://chemistry.about.com/cs/inorganic/a/aa032503a.htm. Retrieved 2009-06-06.
- ^ Reeves, Nicholas Akhenaten: Egypt's False Prophet, Thames & Hudson, p.69 ISBN 0500285527
- ^ "A Case for the World's First Coin: The Lydian Lion". http://rg.ancients.info/lion/article.html. Retrieved 2009-07-24.
- ^ Mansa Musa - Black History Pages
- ^ "Kingdom of Mali - Primary Source Documents". African studies Center. Boston University. http://www.bu.edu/africa/outreach/materials/handouts/k_o_mali.html. Retrieved 2008-08-05.
- ^ Berdan, Frances; Anawalt, Patricia Rieff (1992). The Codex Mendoza. 2. University of California Press. p. 151. ISBN 9780520062344.
- ^ a b "Goldsheet - yearly and cumulative world gold production charts". http://www.goldsheetlinks.com/production2.htm. Retrieved 2006-07-22.
- ^ Moore, Mark A. (2006). "Reed Gold Mine State Historic Site". North Carolina Office of Archives and History. http://www.nchistoricsites.org/Reed/reed.htm. Retrieved 2008-12-13.
- ^ Garvey, Jane A. (2006). "Road to adventure". Georgia Magazine. http://www.georgiamagazine.org/archives_view.asp?mon=7&yr=2006&ID=1344. Retrieved 2007-01-23.
- ^ Seeger, Philip A.; Fowler, William A.; Clayton, Donald D. (1965). "Nucleosynthesis of Heavy Elements by Neutron Capture.". The Astrophysical Journal Supplement Series 11: 121. doi:10.1086/190111.
- ^ "Formation of Lode Gold Deposits". arizonagoldprospectors.com. http://arizonagoldprospectors.com/formation.htm. Retrieved 2009-05-23.
- ^ "Environment & Nature News - Bugs grow gold that looks like coral - 28 January 2004". http://www.abc.net.au/science/news/enviro/EnviroRepublish_1032376.htm. Retrieved 2006-07-22. This is doctoral research undertaken by Frank Reith at the Australian National University, published 2004.
- ^ Kenison Falkner, K.; Edmond, J (1990). "Gold in seawater". Earth and Planetary Science Letters 98 (2): 208–221. doi:10.1016/0012-821X(90)90060-B.
- ^ Plazak, Dan A Hole in the Ground with a Liar at the Top (Salt Lake: Univ. of Utah Press, 2006) ISBN 0874808405 (contains a chapter on gold-from seawater swindles)
- ^ Haber, F. (1927). "Das Gold im Meerwasser". Zeitschrift für Angewandte Chemie 40 (11): 303–314. doi:10.1002/ange.19270401103.
- ^ McHugh, J.B. (1988). "Concentration of gold in natural waters". Journal of Geochemical Exploration 30 (1–3): 85–94. doi:10.1016/0375-6742(88)90051-9.
- ^ Mandaro, Laura (2008-01-17). "China now world's largest gold producer; foreign miners at door - MarketWatch". MarketWatch. http://www.marketwatch.com/news/story/china-now-worlds-largest-gold/story.aspx?guid=%7B8C528CE8%2D0262%2D485D%2DACEB%2D2247D18282CB%7D. Retrieved 2009-04-05.
- ^ Beinhoff, Christian. Removal of Barriers to the Abatement of Global Mercury Pollution from Artisanal Gold Mining. http://www.unido.org/fileadmin/import/10644_CHRISTIANtext.3.pdf.
- ^ Noyes, Robert (1993). Pollution prevention technology handbook. William Andrew. p. 342. ISBN 0815513119. http://books.google.com/?id=__lqGczo9TwC&pg=PA342.
- ^ Pletcher, Derek and Walsh, Frank (1990). Industrial electrochemistry. Springer. p. 244. ISBN 0412304104. http://books.google.com/?id=E_u9ARrm37oC&pg=PA244.
- ^ Marczenko, Zygmunt and Balcerzak, María (2000). Separation, preconcentration, and spectrophotometry in inorganic analysis. Elsevier. p. 210. ISBN 0444505245. http://books.google.com/?id=0NE1KjVISyAC&pg=PA210.
- ^ O'Connell, Rhona (13 April 2007). "Gold mine production costs up by 17% in 2006 while output fell". http://www.mineweb.net/mineweb/view/mineweb/en/page33?oid=19485&sn=Detail.
- ^ "The Myth of the Gold Supply Deficit". http://www.lewrockwell.com/blumen/blumen14.html. Retrieved 2009-03-30.
- ^ estimate of total gold loss over history Accessed Nov. 10, 2010
- ^ "India's love affair with gold tarnishing". March 27, 2008. http://www.nakedcapitalism.com/2008/03/indias-love-affair-with-gold-tarnishing.html.
- ^ "Gold: Why China outbeats India in gold reserves". Commodity online. 2009-04-26. http://www.commodityonline.com/news/Gold-Why-China-outbeats-India-in-gold-reserves-17196-3-1.html.
- ^ Shaw III, C. F. (1999). "Gold-Based Medicinal Agents". Chemical Reviews 99 (9): 2589–2600. doi:10.1021/cr980431o. PMID 11749494.
- ^ Holleman , Wiberg (2001). Inorganic Chemistry (101 ed.). Academic Press. pp. 1286. ISBN 0123526515.
- ^ Jansen, Martin (2005). "Effects of relativistic motion of electrons on the chemistry of gold and platinum". Solid State Sciences 7 (12): 1464–1474. doi:10.1016/j.solidstatesciences.2005.06.015.
- ^ a b Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Wickleder, Mathias S. (2001). "AuSO4: A True Gold(II) Sulfate with an Au4+2 Ion". Journal of Inorganic and General Chemistry 627: 2112–2114. doi:10.1002/1521-3749(200109)627:9<2112::AID-ZAAC2112>3.0.CO;2-2.
- ^ Wickleder, Mathias S. (2007). Francesco A. Devillanova. ed. Handbook of chalcogen chemistry: new perspectives in sulfur, selenium and tellurium. Royal Society of Chemistry. pp. 359–361. ISBN 0854043667. http://books.google.com/?id=IvGnUAaSqOsC&pg=PA359.
- ^ Seidel, S.; Seppelt, K. (2000). "Xenon as a Complex Ligand: The Tetra Xenono Gold(II) Cation in AuXe42+(Sb2F11−)2". Science 290 (5489): 117–118. doi:10.1126/science.290.5489.117. PMID 11021792.
- ^ Riedel, S.; Kaupp, M. (2006). "Revising the Highest Oxidation States of the 5d Elements: The Case of Iridium(+VII)". Angewandte Chemie International Edition 45 (22): 3708–3711. doi:10.1002/anie.200600274. PMID 16639770.
- ^ Dierks, S (May 2005). "Gold MSDS". Electronic Space Products International. http://www.espi-metals.com/msds's/gold.htm.
- ^ Wright, I. H.; Vesey, C. J. (1986). "Acute poisoning with gold cyanide". Anaesthesia 41 (79): 936–939. doi:10.1111/j.1365-2044.1986.tb12920.x.
- ^ Wu, Ming-Ling; Tsai, Wei-Jen; Ger, Jiin; Deng, Jou-Fang; Tsay, Shyh-Haw; Yang, Mo-Hsiung. (2001). "Cholestatic Hepatitis Caused by Acute Gold Potassium Cyanide Poisoning". Clinical toxicology 39 (7): 739–743. doi:10.1081/CLT-100108516. PMID 11778673.
- ^ Henna tattoo ingredient is Allergen of the Year.(Clinical Rounds). Retrieved Sept 17, 2009.
- ^ Brunk, Doug (February 15, 2008). "Ubiquitous nickel wins skin contact allergy award for 2008". http://www.highbeam.com/doc/1G1-176478357.html.
- ^ "World Gold Council > value > research & statistics > statistics > supply and demand statistics". http://www.gold.org/value/stats/statistics/gold_demand/index.html. Retrieved 2006-07-22.
- ^ Kitco.com, Gold - London PM Fix 1975 - present (GIF), Retrieved 2006-07-22.
- ^ "Goldfinger Brown's £2 billion blunder in the bullion market". The Times (London), 15 April 2007.
- ^ a b "LBMA statistics". Lbma.org.uk. 2008-12-31. http://www.lbma.org.uk/2008dailygold.htm. Retrieved 2009-04-05.
- ^ "Gold hits yet another record high". BBC News. 2009-12-02. http://news.bbc.co.uk/2/hi/business/8390779.stm. Retrieved 2009-12-06.
- ^ "PRECIOUS METALS: Comex Gold Hits All-Time High". The Wall Street Journal. May 11, 2010. http://online.wsj.com/article/BT-CO-20100511-717954.html. Retrieved August 4, 2010. [dead link]
- ^ Gibson, Kate; Chang, Sue (May 11, 2010). "Gold futures hit closing record as investors fret rescue deal". MarketWatch. http://www.marketwatch.com/story/gold-prices-resume-rise-as-eu-plan-pondered-2010-05-11. Retrieved August 4, 2010.
- ^ 10 Year Gold (GIF). Kitco.com.
- ^ "Gold starts 2006 well, but this is not a 25-year high!". Ameinfo.com. http://www.ameinfo.com/75511.html. Retrieved 2009-04-05.
- ^ California Government Code selection 420-429.8 (see § 425.1)
- ^ Alaska Statutes (see§ 44.09.110)
External links
| Wikimedia Commons has media related to: Gold |
| Look up gold in Wiktionary, the free dictionary. |
- Getting Gold 1898 book, www.lateralscience.co.uk
- Technical Document on Extraction and Mining of Gold, www.epa.gov
- Picture in the Element collection from Heinrich Pniok, www.pniok.de
- WebElements.com — Gold n www.webelements.com
- Chemistry in its element podcast (MP3) from the Royal Society of Chemistry's Chemistry World: Gold www.rsc.org
| |||||||||||||||||||||||||||||||||||||||
| [hide] Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| | |||||||||||||||||||||||||||||||||||||||||
Labels:
gold
Subscribe to:
Comments (Atom)